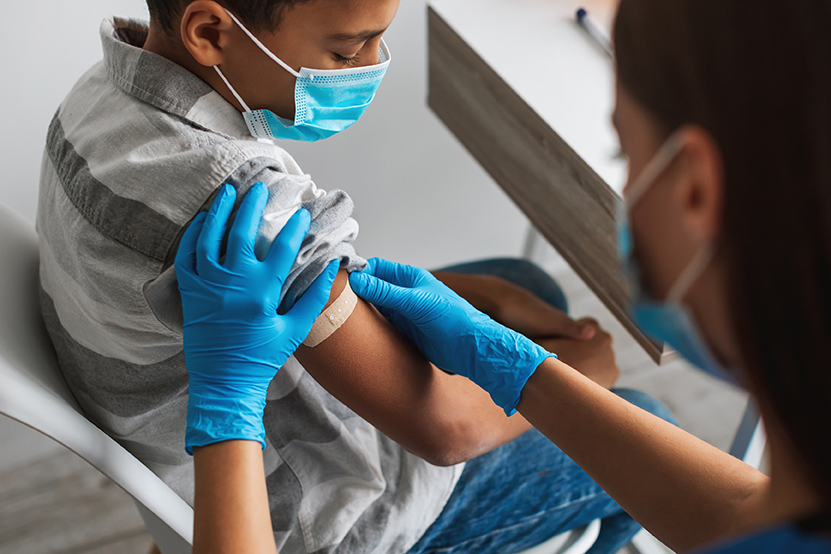
Medicines for Malaria Venture (MMV) is a nonprofit organization that works with partners to discover, develop and deliver new, effective, and affordable antimalarial drugs. One of MMV’s current projects is to develop a long-acting injectable product to protect children at risk of malaria. To ensure the product would achieve its goals, MMV needed clear guidance on what was most important to clinicians treating pediatric patients in malaria endemic countries. Getting there required understanding the business and real-world clinical side of taking a health product to market. This is familiar territory for WDI’s Healthcare team.
Early Answers for Effective Drugs
To find the best path forward for its injectable, MMV sought answers from the drug’s final users. As it updated the injectable’s Target Product Profile (TPP)[CA1] , which is a compilation of the desired characteristics that will make up a product or device, MMV wanted to know what aspects of the new product would create the most value for clinicians administering it to children.
“When you develop a product, there are a number of elements that you can act on, and we wanted to know, from the local stakeholders, that is the people in the field, the decision-makers in the countries, what matters to them,” said Céline Audibert, Director of Market Research, Access & Product Management at MMV.
Audibert enlisted WDI’s help to better understand those elements. WDI’s Healthcare group wrote and carried out a survey in six African countries — some of the places that would benefit most from such a product. It was sent to healthcare professionals and public health officials, including those in charge of maintaining nationwide malaria-related efforts, in Cameroon, the Democratic Republic of Congo, Senegal, Ghana, Mali, and Nigeria. The survey covered a series of characteristics in an injectable product, including duration of protection, efficacy, number of injections, volume per injection, needle size, and tolerability. It laid out various options with different levels of each characteristic and asked participants which ones they would choose.
This method of choosing various combinations of parameters, rather than simply asking for a ranking, was intentional. It forced a choice, thereby providing a deeper understanding of the importance of various elements. When a large enough group of participants are pushed to make trade-offs, researchers can uncover what truly matters to the population.
Uncovering preferences for the product characteristics was the objective of the study — and key to developing an injectable that would be both adopted and effective. “In the past, there wasn’t a focus on the needs of a particular group of people, at the country or regional level,” said Pascale Leroueil, Vice President of Healthcare, who led the research. “The more we build Target Product Profiles specific to the countries where we’re introducing new products, the better.”
Pulling Private-Sector Factors into Public-Sector Work
WDI didn’t need to reinvent the wheel to develop its survey and methodology. The Institute has been gathering and analyzing data for decades to support business-centered solutions that drive economic growth and social freedom in low- and middle-income countries. In this case, it applied a well-tested system in a space where it had been rarely used.
“There are tried-and-true methods that are utilized in the private sector that we can apply to this area, and WDI does that better than most,” said Leroueil.
Leroueil and Ben Davis, WDI Senior Research Fellow, proposed the methodology to Audibert, who was familiar with it from her previous private-sector work. They both understood the core purpose behind the answers they sought. “There are reasons people want something,” explained Leroueil. Supply issues, cultural preferences, and geographic needs can drive the acceptance or denial of treatment — and no amount of after-the-fact adjustments will bring the same benefits as early-stage consideration.
The results were promising. “This research has confirmed some of the things we believe. There were characteristics where we had a lack of understanding internally and needed information from the field, so we had very clear answers on what matters and what doesn’t,” Audibert said.
The Value of Investing at the Start
The value of getting these answers at the start of the development process is broad and inclusive. Investors and donors can be sure their money is being put to effective use, drug development teams can focus their energy on the aspects of a product that matter most, and patients can get the drugs that serve them best.
Efficiencies in development matter to nonprofits like MMV. “When you have a restricted amount of money to invest, you need to know where you’re going to put that money,” said Audibert. MMV is currently working on funding for the continuation of the project, so this information will be used, in part, to demonstrate how the injectable can better meet the needs of the populations they plan to serve.
The research also makes a major difference for scientists, developers, and those in the business of drug delivery. Audibert expects that having these answers will help optimize the time and investment spent on drug development and support better-informed decisions. With a focus on what counts, researchers can create the best possible product for the population.
Finally, patients are more likely to accept treatments that fit their needs, and that acceptance can support healthier families, communities, and countries.
“Businesses that know how their customers will use their products usually have the edge over those that wait for feedback after a product is launched,” Leroueil said. “It’s exciting to see organizations like MMV take this approach.”